

Antimicrobial resistance (AMR) is a complex, global public health threat that affects veterinary and human medicine.
Many infectious diseases are caused by bacteria and can be treated with antibiotics.
Antimicrobial resistance occurs when bacteria no longer respond to antibiotics – as these drugs lose their effectiveness, infectious diseases become harder to control.
Antibiotics are commonly administered to target the bacterial component of bovine respiratory disease (BRD) complex.
Practical strategies to monitor AMR in beef cattle are generally lacking in the United States.
Veterinary diagnostic laboratories have reported increasing trends of antibiotic resistance, often sampled from animals that die of pneumonia.
It is unclear how well these reports reflect the general cattle population and what level of resistance exists among live animals.
This article summarizes the findings of a PAC research study completed in partnership with Zoetis and Boehringer Ingelheim Animal Health.

This study sampled calves at the first diagnosis of BRD from 15 commercial US feedlots.
Deep pharyngeal swabs were collected from 453 calves. The swabs were cultured at a diagnostic lab to identify bacteria associated with respiratory disease.
These bacteria were then subjected to antimicrobial susceptibility testing that indicated if the bacterial isolate was susceptible, intermediately resistant, or resistant to different antibiotics.
In this population of calves, 17.2% received injectable metaphylaxis and 74.2% received chlortetracycline (CTC) in the feed.
Wide ranges of days on feed (1 to 253 DOF) and weight (306 to 1574 lbs.) at treatment were observed. All calves received antibiotic treatment following sampling.
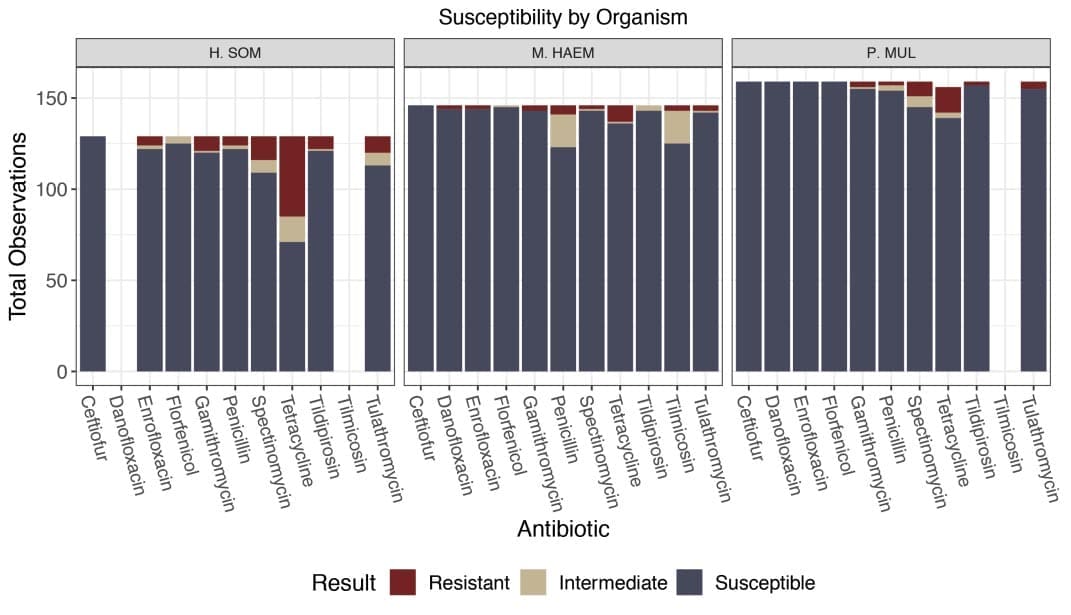
Three main species of bacteria were isolated from culture and include 28.5% Histophilus somni, 32.7% Mannheimia hae molytica, and 36% Pasteurella multocida.
Of the H. somni isolates, 39.5% were resistant to at least one antimicrobial.
Multidrug resistance (MDR) occurs when an isolate is not susceptible to at least one drug across three or more antibiotic classes.
By this definition, 9.3% of Histophilus isolates were multi-drug resistant.
Mannheimia isolates totaled 8.8% resistance to at least one antimicrobial and 2.7% multidrug resistant.
Resistance in Pasteurella isolates totaled 11.7% resistant to at least one antimicrobial and 1.8% multidrug resistant.

Antimicrobial susceptibility results were analyzed with animal outcomes and region of the feedlots sampled, in addition to the regions of calf origin.
No significant trends in AMR were observed by geographical regions.
Evidence did not support any changes in animal outcomes with resistance prevalence.
Overall, 88% shipped to slaughter with their respective lot, 4.9% deviated from lot shipment date, and 7.1% died.
First treatment success was 70.2%. Of the mortalities, the BRD case fatality rate was 6.4%.
The highest resistance in this study was to tetracycline (Figure 1).
This resistant population was further explored with a statistical analysis.
In all three bacteria, resistance to tetracycline was 5.7 times more likely in animals that received metaphylaxis compared to those that did not (p < 0.0001).[1]
Of the metaphylaxis group, no significant difference was detected in the proportion of resistance among animals requiring retreatment (p = 0.1263).[1]
Days on feed was divided into 5 groups and the tetracycline-resistant Histophilus was most likely to occur between days 21 to 60.[1]
Antimicrobial susceptibility testing is a tool to guide treatment decisions and does not guarantee a specific clinical result in an individual animal.
Ultimately, disease outcomes are driven by a variety of host factors such as immune status, genotype, stress, disease severity and duration, etc., which were not accounted for in this study.
This study describes antimicrobial resistance that exists at initial diagnosis of BRD using upper respiratory tract samples.
Other studies report higher levels of resistance and multidrug resistance from lower respiratory tract samples[2] (trans-tracheal washes) and lung tissue from terminal cases.[3]
A study published by Iowa State University reported the percentage of resistant isolates increased as the number of antimicrobial treatments increased.[4]
Previously untreated animals demonstrated MDR in less than 5% of total samples, compared to 47% from animals with three or more antibiotic treatments.[4]
These studies describe changes occurring as sick animals fail to recover and support evidence that cautious use of antibiotics is a necessity.
1. Jobman, E., Hagenmaier, J., Meyer, N., Harper, LB., Taylor, L., Lukasiewicz, K., Thomson, D., Lowe, J., Terrell, S. Cross-section observational study to assess antimicrobial resistance prevalence among bovine respiratory disease bacterial isolates from commercial US feedlots. Antibiotics. 2023; 12(2):215.
2. Timsit, E., Hallewell, J., Booker, C., Tison, N., Amat, S., Alexander, T. Prevalence and antimicrobial susceptibility of Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni isolated from the lower respiratory tract of healthy feedlot cattle and those diagnosed with bovine respiratory disease. Vet. Microbiol. 2017, 208, 118–125.
3. Lubbers, B., Hanzlicek, G. Antimicrobial multidrug resistance and coresistance patterns of Mannheimia haemolytica isolated from bovine respiratory disease cases – A three-year (2009–2011) retrospective analysis. J. Vet. Diagn. Investig. 2013, 25, 413–417.
4. Magstadt, D., Schuler, A., Coetzee, J., Krull, A., O’Connor, A., Cooper, V., Engelken, T. Treatment history and antimicrobial susceptibility results for Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni isolates from bovine respiratory disease cases submitted to the Iowa State University Veterinary Diagnostic Laboratory from 2013 to 2015. J. Vet. Diagn. Investig. 2018, 30, 99–104.
 Erin Jobman grew up on a farm in central Nebraska where her family continues to raise food-grade corn and cattle. She joined the PAC team as a research veterinarian. Previously, she worked as a mixed animal practitioner before joining the Great Plains Veterinary Educational Center for several years. She is a graduate of Kansas State University (DVM and MPH) and the University of Nebraska-Lincoln (BS Animal Science). Erin serves on the Board of Health for the South Heartland District Health Department. She and her husband, Ben, reside in south-central Nebraska where they enjoy all things related to sports and the great outdoors.
Erin Jobman grew up on a farm in central Nebraska where her family continues to raise food-grade corn and cattle. She joined the PAC team as a research veterinarian. Previously, she worked as a mixed animal practitioner before joining the Great Plains Veterinary Educational Center for several years. She is a graduate of Kansas State University (DVM and MPH) and the University of Nebraska-Lincoln (BS Animal Science). Erin serves on the Board of Health for the South Heartland District Health Department. She and her husband, Ben, reside in south-central Nebraska where they enjoy all things related to sports and the great outdoors.
Get all Doc Talk episodes straight to your email inbox!