

We have all been frustrated with infectious bovine keratoconjunctivitis (IBK, “pinkeye”) at least once.
Given our dedication to our clients and our commitment to evidence-based medicine, when frustrated by IBK, we go to textbooks, the published literature, etc. to try to find the answers.
Unfortunately, while IBK has been studied for more than a century, there is still much to be learned. Discouraged by both lack of definitive answers and sometimes conflicting answers, we turn to our experience.
However, in our experience, we have seen huge problems with IBK in a given year or on a given operation, and little to no problem the next year or on the neighboring operation.
Unfortunately, it is not at all clear what was different or what we did or did not do to lead to the difference. If you have been discouraged by the lack of answers and conflicting answers, keep reading.
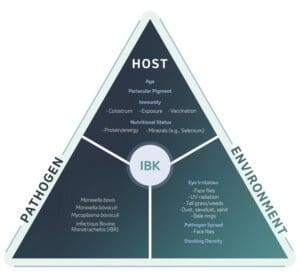
The disease triangle (host – pathogen – environment) is particularly applicable to pinkeye, and all three are equally important and must be considered in any prevention plan (see Figure 1)
Damage to the cornea is a necessary first step in the pathogenesis of pinkeye. The importance of this cannot be overstated.
Female face flies (Musca autumnalis) feed on ocular secretions and have microscopic sharp mouthparts that damage ocular tissues to stimulate production of these secretions.
Moraxella bovis, Moraxella bovoculi, and Mycoplasma bovoculi are commensals of the bovine eye. All three are pretty much always there.
There is abundant, though interestingly not conclusive, evidence to support the generally accepted view that Moraxella bovis causes IBK.
Abrading or otherwise damaging (e.g., using ultraviolet radiation) the bovine cornea followed by instillation of a pathogenic strain of M. bovis consistently leads to IBK.
The pathogenesis of M. bovis-associated IBK is a three-step process: 1. corneal damage, 2. microbial adhesion facilitated by attachment pili, 3. release of cytotoxins by M. bovis that rapidly “melt” the cornea in as little as 48 hours.
Some M. bovis vaccines are directed against the attachment pili. Unfortunately, M. bovis is adept at switching surface pili and antibody pressure can induce pilus switching, leading to apparent vaccine failure.
An obvious solution would thus be to build a vaccine containing many different pilus epitopes. However, the inclusion of “too many” different pili leads to antigenic competition.
That is, the immune system would “see” so many antigens that it would only respond to some. How many is too many is not yet well-defined.
O’Connor et al (JVIM 2011) reviewed nine trials of autogenous IBK vaccines and concluded that “autogenous M. bovis vaccines … are ineffective in controlling naturally occurring IBK.”
M. bovoculi is commonly cultured from the eyes of affected cattle and has been associated with IBK. It is important to remember that association does not prove causation.
The standard disease model (damaging the cornea followed by instillation of bacteria) does not lead to IBK when M. bovoculi is used. Indeed, there is not a disease model for IBK caused by M. bovoculi.
That is not to say that Moraxella bovoculi is not involved in the pathogenesis of some cases of IBK, but simply that the pathogenesis of IBK potentially caused by M. bovoculi is not fully understood.
Infectious bovine rhinotracheitis (IBR, BHV-1) is associated with a pinkeye like syndrome. There is some evidence that vaccination with modified live IBR vaccine may be a risk factor for IBK.
Elimination of ocular M. bovis infection depends more on reaching therapeutic drug concentrations in the infected ocular tissues than in tears.
Oxytetracycline is selectively concentrated in ocular tissues including the epithelium of the conjunctiva and lacrimal gland ductules, reaching concentrations in these tissues that exceed those in serum.
Electron microscopy studies suggest that M. bovoculi may attach poorly to the corneal surface but attach well to M. bovis. Furthermore, M. bovoculi growth may outpace that of M. bovis.
This suggests a hypothetical scenario wherein M. bovis attachment to damaged cornea (via pili) is necessary to “get things started” followed by Moraxella bovoculi “taking over.”
If true, then this implies both that timing of ocular culture is critically important vis-à-vis interpretation of culture results, and that vaccination against M. bovis should be sufficient to prevent disease.
Pilus switching by M. bovis may occur between the time of isolate submission for development of an autogenous vaccine and the time of vaccination.
Given that M. bovis, M. bovoculi, and M. bovoculi are commensals of the bovine eye, why do we have apparent outbreaks of IBK?
That is, why does the disease seem to be contagious? Is it simply that environmental conditions led to corneal damage in many individuals, or is a more pathogenic variant being spread from animal to animal, or both?
What is more important toward immunity to IBK, IgG or IgA? That is, might intranasal or intraocular vaccination be more effective?
Is M. bovoculi important, and if so, how and under what circumstances does it fit in to the IBK picture?
Considering that the typical IBK season begins when maternal antibody may interfere with vaccination for spring born calves in the northern USA, when should we vaccinate?
Similarly, should extensively managed calves that are only handled once prior to weaning be vaccinated at branding?
Decrease corneal damage. While easy to say but less easy to do, remember that difficult is not the same as impossible. A story may help to illustrate.
During a visit to a large heifer development facility in the southwest U.S. where the herdsman was complaining of an ongoing IBK problem, scraping and hauling of manure from a pen nearly half a mile away was occurring.
Trucks were driving on the dirt road along the western border of the facility every two to three minutes. As I visited with the herdsman, my eyes were stinging from the dust.
The herdsman stated that even when pens were not being scraped, the road was heavily traveled.
This operation did not need a different or better vaccine, it needed a water truck to water the road. There is a reason that “clip pastures and control flies” appears in every IBK article.
Evaluate each operation individually to identify and stratify sources of corneal damage and then work to minimize them.
Ensure that animals are prepared for the challenge. Minimize stressors, including parasitism.
Provide adequate protein, energy, vitamins, and minerals. If possible, prepare the host with two doses of commercially available M. bovis (regardless of label) and M. bovoculi vaccine.
 Dr. Lowell Midla is a technical services veterinarian for Merck Animal Health. Prior to joining Merck Animal Health, he served 24 years as a practicing veterinarian and 15 years teaching at Ohio State University Veterinary School.
Dr. Lowell Midla is a technical services veterinarian for Merck Animal Health. Prior to joining Merck Animal Health, he served 24 years as a practicing veterinarian and 15 years teaching at Ohio State University Veterinary School.
Notifications